Editor’s Note: Vital Signs is a monthly program bringing viewers health stories from around the world.
They grow up to one meter in length and kill their prey using injections of venom.
Once the venom takes effect, victims black out from a drop in blood pressure, leaving them trapped and ready to be eaten – head-first.
This is the feeding habit of the Brazilian pit viper, a snake found in the Amazon basin and forests of Brazil, whose venom is the source of one of the most commonly prescribed drugs for hypertension (abnormally high blood pressure) – captopril.
“This [snake venom] opened up a completely new class of medication,” says Zoltan Takacs, founder of the World Toxin Bank project, referring to a class of medications known as angiotensin-converting enzyme (ACE) inhibitors, now used to treat more than 40 million people worldwide.
Approved by the FDA in 1981, captopril is not a new drug, but its approval pushed the idea that venoms could be used to create modern medicines.
The field of venom-based medicine has flourished ever since and teams across the world are now exploring the most remote of animals in search of potent drugs that could emerge from their highly evolved venom.
Read: This man’s blood has saved the lives of two million babies
Since captopril, two more drugs – eptifibatide and tirofiban – based on venoms from the dusky pygmy rattlesnake and saw-scaled viper, respectively, were approved in the late 1990s to treat other heart conditions, such as angina.
“For the main types of heart attack [in the United States], there are three drugs and two come from snake venom,” says Takacs.
Worldwide, up to 100,000 fatalities are estimated to occur as a result of venomous snake bites each year. But snakes are also making their mark on human health in contrast to the way nature intended – by saving lives.
Learning from evolution
“Venoms have evolved to immobilize or kill …They target vital bodily functions,” says Takacs.
When used by animals, venoms are tailored to target one of two vital functions within the body – blood circulation or the communication between nerves and muscles. Their purpose is to injure muscle, numb nerves, or stop blood from clotting so their victims bleed endlessly, making it easier for the predator to eat their now-stranded prey.
But these effects on the body also have the potential to be beneficial – through alleviating pain or the prevention of blood clots – and are the prime goals of drug discovery companies in their quest to cure or treat diseases such as heart attacks or neurological disorders.
“They target the right molecules within the human body in order to cure a disease … this makes them ideal drug leads,” says Takacs.
The field as a whole spans beyond lethal snakes to other venom-producing animals such as leeches, cone snails, scorpions and lizards. But as snakes primarily target warm-blooded animals, their venoms are more fruitful for human drugs.
Read: The worms that invade your brain
Seven drugs derived from animal venom have been approved by the FDA to date to treat conditions ranging from hypertension and other heart conditions to chronic pain and diabetes. Ten more are in clinical trials and even more in pre-clinical stages awaiting tests for safety and then trials in humans.
But the route from venom to drug is a long and arduous process – with the risk to researchers of bites and stings along the way.
Finding a drug from a snakepit
The new drugs are not based on the venoms themselves, but instead on one of the many toxins found within them.
“Venom is a cocktail of natural toxins [and] can contain as little as 20 to 30 toxins [and up] to 100 toxins,” says Kini R Manjunatha, professor of biological sciences at the National University of Singapore, whose team work with 70 to 100 snake venoms at any one time.
“It depends on the novelty of the venom,” he explains.
When a venom is found to have a beneficial effect on the body – such pain relief or preventing blood clots – it’s broken down into its constituent toxins, which are studied to identify first their structure and then the relevant receptors on human cells where they work.
Being selective
The advantage of exploring specific toxins is how selective they are when attaching to their targets within the body – minimizing the potential for unwanted side effects.
“Toxins have evolved for millions of years to target a specific receptor,” says Manjunatha. Also important is their potency, as just a small amount can have fatal effects.

Manjunatha’s team are currently working with venom from the king cobra, from which they’ve isolated a particular toxin with strong potential as a treatment for chronic pain due to its analgesic – painkilling – effects on the body. The team manipulated the toxin’s ability to act on the central nervous system to produce a drug capable of reducing sensitivity to pain. They say their trials in mice have shown painkilling effects 20 times greater than morphine and with zero side-effects so far.
“Currently, one of the best analgesic [drugs] available is aspirin, [but] it has side effects … for chronic use this is a problem,” says Manjunatha.
It’s early days, as tests have so far only been conducted on animals, but after further safety trials they hope to soon experiment on humans.
Getting into your blood
As well as painkillers and treatments for other neurological conditions, further drugs are also in development for stoke and cardiovascular disease as well as conditions such as prostate cancer, HIV and multiple sclerosis.
“We’re interested in the venoms that bind to platelets,” says professor Bryan Fry, from the University of Queensland, Australia. Platelets are the components of blood that help it to clot and by preventing unnecessary blood clots, conditions such as heart attacks can be avoided. Known as anticoagulants, this form of drug can also be useful during surgery to prevent blood from clotting.
Fry is also working with more than 100 snake venoms in his lab to try to stop blood clotting – including that of the Iranian spider-tail viper. “It’s potent but very precise,” says Fry.
The mechanism behind the venom is yet to be understood but Fry sees potential as his targeted toxin within the venom is small in size, making it is less likely to be recognized by the body’s immune system when used – and less likely to be attacked.
“You’re at less risk of an allergic reaction,” explains Fry.
Now they’ve identified it, the next steps are to understand how it works and then design a synthetic version for use as a drug.
“If you can get it into a pill, that’s your goal,” he says. But with such a goal comes the challenge of a drug surviving the body’s digestive system and stomach acids to be absorbed into the body and make its mark.
Beyond the bite
But Fry is determined, despite having faced the wrath of an angry snake on numerous occasions. He has suffered 24 snake bites to date, including that of the Iranian viper, whose venom is fueling his latest drug.
“I was bleeding out of my eye,” he recalls of the experience. A sting from a scorpion once left his heart stopping every 30 seconds until he was treated, but the adventurous scientist has now upped the challenge to work with Komodo dragons as well.
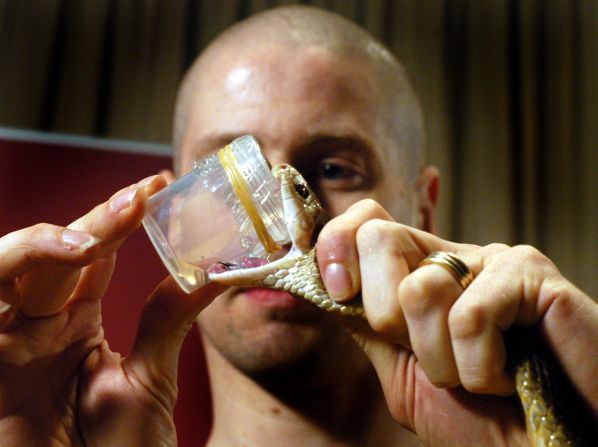
Fry still ventures into the field himself to “milk” snakes for their venom – which involves luring snakes to bite onto a material laid over the opening of a jar that captures their venom.
“Snakes are the easiest to get venom out of,” he says.
Whilst his research also remains in the early stages of development, Fry is hopeful one of his many current studies will result in something benefiting humankind.
But new drugs will take time.
“It takes seven to 25 years to develop a drug once you identify a toxin,” says Takacs, whose Toxin Bank is a tool for researchers to use as a library to see how toxins may work together and create a more potent and selective drug.
“People are always going to get sick, so you need new cures … snakes and other creatures can give you this life-saving medicine, he says. “Twenty million toxins remain unexplored in nature.”