Story highlights
The committee continues to advise that everyone 6 months and older get vaccinated
All women who are pregnant or may become pregnant should get the flu shot, panel says
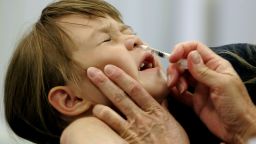
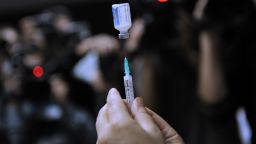
Shots will continue to be the main option for the upcoming flu season. A US Centers for Disease Control and Prevention advisory committee recommended Wednesday that FluMist, the nasal spray influenza vaccine, not be used during the 2017-18 season.
Though it’s popular among those who hate needles – including most children – last year’s recommendation to exclude FluMist did not affect vaccine coverage numbers for the 2016-17 season compared with the 2015-16 season according to preliminary data presented to the committee. Overall, 58.2% of US children between the ages of 6 months and 17 years were vaccinated, compared with 59% the previous year.
The US Food and Drug Administration first approved the nasal spray, produced by MedImmune, a subsidiary of London-based AstraZeneca PLC, in 2003. By all accounts, it worked well in the early years. Last season, though, the CDC’s Advisory Committee on Immunization Practices decided to not recommend the spray because of its poor performance compared with the flu shot.
At last year’s meeting, MedImmune’s Dr. Chris Ambrose shared results from the company’s 2015-16 effectiveness study, which found the FluMist quadrivalent vaccine to be 46% effective, compared with the flu shot’s 65% effectiveness.
In response to these findings, AstraZeneca initiated a scientific investigation to identify potential causes of lower effectiveness, explained Alexandra Engel, director of media relations for the company, in an email. She said discussions continue with the FDA, the CDC and the advisory committee with “a goal of a renewed recommendation” for use during the 2017-18 season.
Although FluMist is not recommended for the upcoming season, the vaccine is still approved by the Food and Drug Administration for people between the ages of 2 and 49. So patients can get it if they find a health care provider offering it, though it might not work as well as a shot.
Wednesday’s presentations at the committee’s meeting also revealed that 99 children died due to the flu during the 2016-17 season, up from 92 the year before.
“If the data are similar to the data presented in previous years, the vast majority of these (pediatric deaths) will be unvaccinated children,” said Dr. William Schaffner, representative on the committee’s Influenza Working Group. “That is my prediction.”
Schaffner said the most important outcome of Wednesday’s meeting was the committee’s decision to reaffirm its standard recommendation that everyone 6 months and older get vaccinated.
As they do every year, the committee of 15 immunization experts reviewed data from previous flu seasons to make its recommendations, which this year include advice that doctors not give their patients FluMist.
Preventing many cases but not all
Overall, the flu vaccine was 61% effective among children between 6 months and 8 years old and 42% across all age groups during the 2016-17 season, Dr. Jill Ferdinands of the CDC’s Influenza Divisionsaid Wednesday. For the oldest age group, those 65 and older, the vaccine was 25% effective.
Still, the vaccine prevented nearly 30% of hospitalizations that might have been caused by flu among Americans of all ages, and that rate was higher – 37% – among adults 65 or older in particular. Overall, the vaccine reduced outpatient visits by 42% for influenza A and B viruseslast season.
Schaffner said the evaluation of vaccine effectiveness has been consistent with previous information from previous years. “Pretty good but not great: That’s the story of flu vaccine, year in, year out. Although we can’t guarantee it will prevent every case of flu, it still prevents many.”
He noted that the vaccination numbers do not account formodified cases, when someone gets the flu but does not suffer too much.
“You got flu, but you weren’t hospitalized, and you didn’t die,” Schaffner said.
Alicia Budd, a member of the CDC advisory committee, noted that influenza A strains predominated last year, with overall moderate flu activity at a similar severity to previous seasons. The season peaked in mid-February, with Western regions showing a peak during January.
Most notably, the majority of circulating viruses were similar to the viruses used to make the vaccines.
An emphasis on pregnant women
Each February, a government committee makes the final decision about which virus strains will go into vaccines sold in America for the coming flu season. Its decision is based on information from more than 100 countries, where influenza-monitoring centers conduct surveillance of circulating viruses. The committee considers which viruses are making people sick, where those viruses are spreading and how well the previous season’s vaccine protects against them.
After the committee selects the strains to be used for the vaccine, manufacturers genetically adapt the strains to optimize the vaccine for the production process. Typically, trivalent formulations include two A strains and one B strain, and quadrivalent formulations add a second B strain.
Join the conversation
The flu shot is an inactivated influenza vaccine, but FluMist is a live attenuated influenza vaccine, which means the live viruses have been weakened (attenuated, in medical terms) and work by stimulating the immune system.
On Wednesday, the committee also approved changes to its current recommendations regarding pregnant women. Though the language will be finessed, it recommends that all women who are pregnant or who might become pregnant during the upcoming influenza season receive the vaccine.
Although pregnant women should not receive live attenuated virus vaccines, any licensed, recommended and age-appropriate trivalent or quadrivalent inactivated vaccine formulation may be used.
In the fine print, the committee recommendation explains that there are a lack of data on safety of approved inactivated vaccines during pregnancy. A public commentator expressed the opinion that flu vaccination be recommended only for women during the second and third trimesters, but the committee maintained its advice that all women who are or might be pregnant get the shot.
Schaffner said the most notable point was the recommendation that everyone 6 months and older get vaccinated. “Parents, please make sure your children are vaccinated,” he said.