Story highlights
A new study suggests that a hominin is to blame for modern genital herpes
It is the most likely culprit to help the disease jump the species barrier
Herpes has been around a long time, to say the least.
Ancient chimpanzees genetically passed oral herpes (herpes simplex 1, or HSV-1) to the earliest humans millions of years ago when our lineage split. And we almost missed out on catching that other scourge, genital herpes (HSV-2) – almost. Unlike HSV-1, HSV-2 didn’t make the leap to early humans on its own.
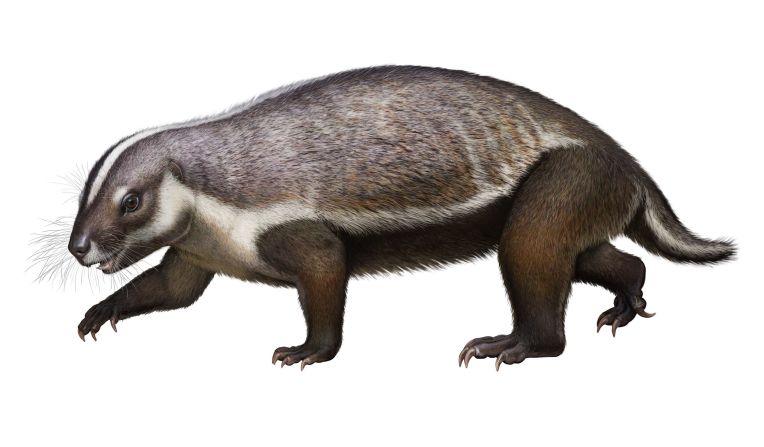
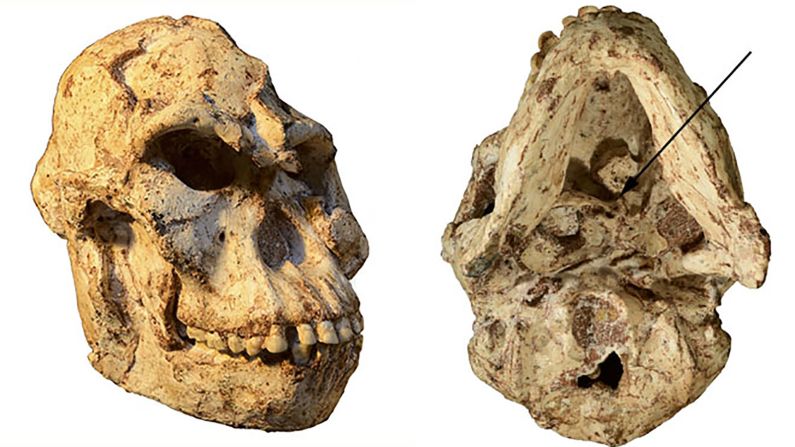
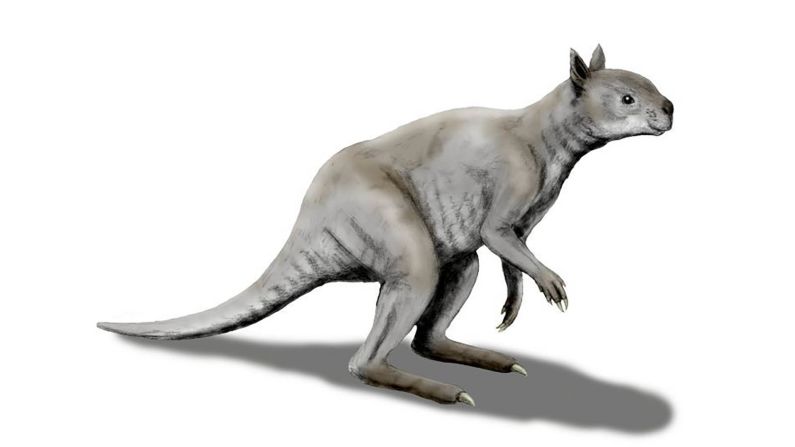
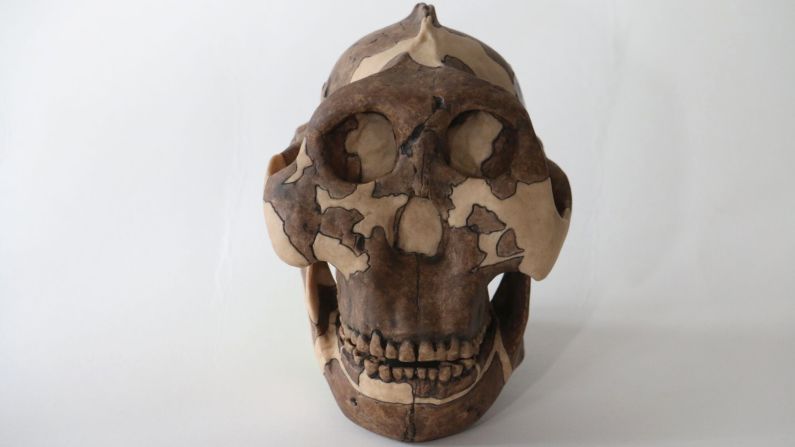
Unfortunately for modern humans, millions of years ago, an early human ancestor was in the right place at the right time to catch HSV-2. And it might not have happened if it weren’t for that meddling hominin species Paranthropus boisei, according to a new study in the journal Virus Evolution.
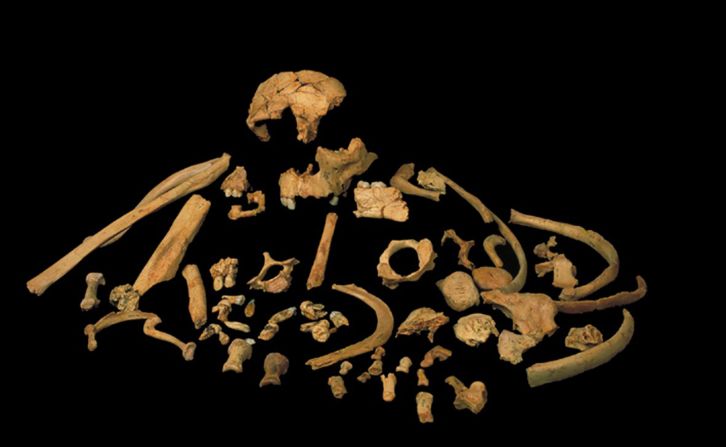
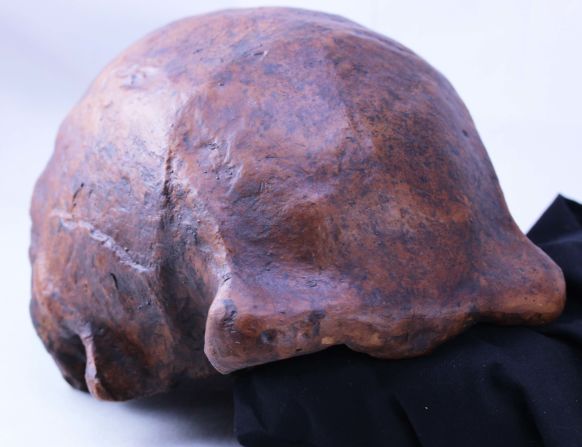
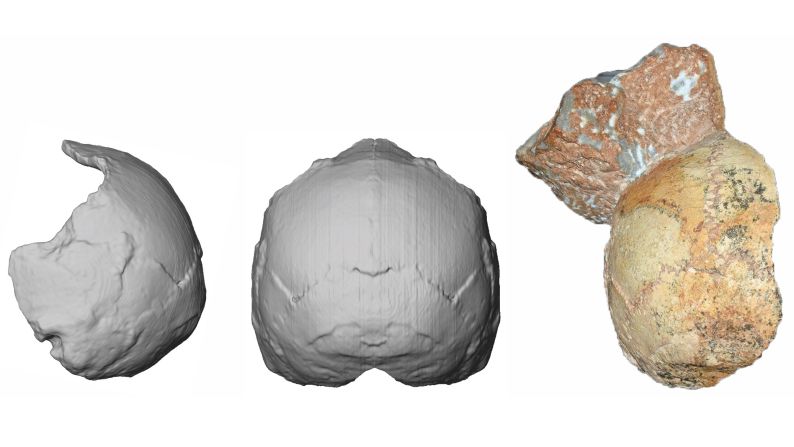
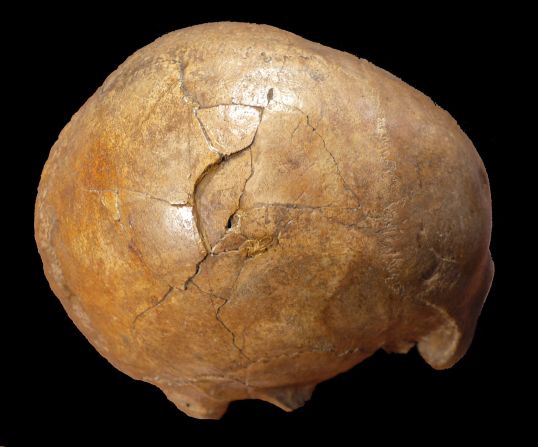
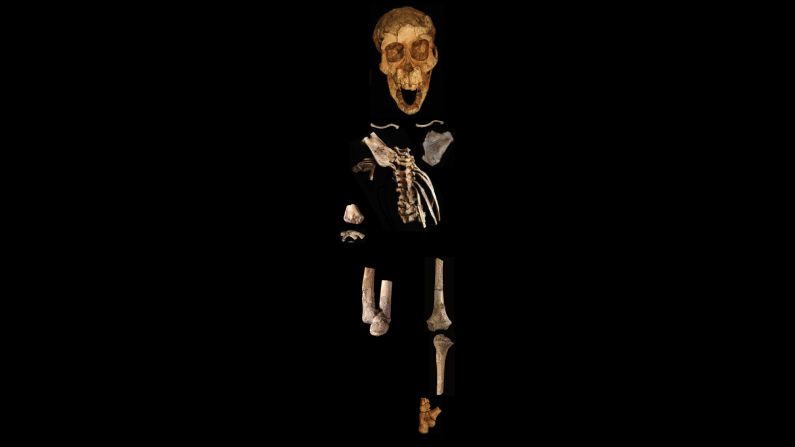
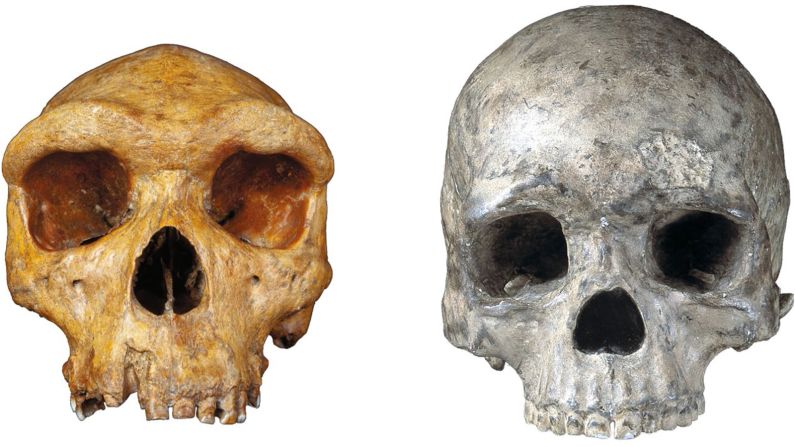
Standing at about 4 feet tall, boisei had a small brain and a wide, dish-like face. It is most well-known for having big teeth and hefty chewing muscles. One skull was nicknamed “Nutcracker Man” for these traits.
Boisei acted as the in-between agent for genital herpes to make the species jump from primates to humans, researchers say.
Ancient chimpanzees, boisei and Homo erectus were all in Africa between 1.4 million and 3 million years ago, in an area where the evolution of modern humans would occur. It would be easy for them to come into contact with each other around water sources.
HSV-2 itself was evolving at the time, and it could be spread orally.
The researchers believe that the circle of life caused this transmission, which would have to happen through fluid exchange. Chimpanzee bites or scratches would transfer the virus through sores.
So what about the human bloodline? According to the researchers, transfer could have happened through sexual intercourse or Homo erectus consuming boisei – or both.
“We can ‘blame’ our ancestors for eating other hominins/great apes, this has been the source of other primate-to-human infections such as HIV,” Charlotte Houldcroft, senior study author and virologist at the University of Cambridge Department of Archaeology, wrote in an email. “Eating other species closely related to oneself has risks, because pathogens adapted to species genetically similar to us will find it easier to jump the species barrier.”
The most genetically diverse strains of HSV-2 come from Africa, and the amount of worldwide HSV-2 genetic diversity points to an out-of-Africa migration, according to the researchers.
“Essentially, when humans migrated out of Africa, they already carried HSV-2, and wherever humans went, their viruses went too,” Houldcroft said. “HSV-2 infects for life and can be passed from mother to baby or between sexual partners, which made sure it successfully spread wherever humans did.”
But making this determination when there are gaps in the fossil record required more than just an analysis of old bones.
“It’s a really interesting question that is invisible to archaeology,” Houldcroft said. “Why wouldn’t you want to try and solve the mystery?!”
The study brought together a virologist, an engineer and a researcher of human evolution to apply and develop a model to meld their areas of expertise.
They used Bayesian network modeling – a graphical model using probability, random variables and conditional dependencies – to combine data of ancient climates, herpes DNA and fossils and determine the probability of HSV-2 and how it might be transmitted.
“We can use data from diseases to reconstruct events that are completely invisible to the archaeological and fossil records,” Houldcroft said. “The signals in the HSV-2 virus are records of direct contact between the ancestors of us and chimps that we can tangibly now ‘see’ and gives us direct insight into the daily lives of our ancestors.”
The researchers said the biggest surprise was not the findings themselves but rather the discovery that their diverse backgrounds informed a different way to look at a problem and solve it creatively.
“Their combined use of bayesian models and AI to fit climate and environmental data with hominin fossils is insightful,” Angelique Corthals, forensic anthropologist and assistant professor at the City University of New York, wrote in an email. “It allows the research to redefine the jump of HSV-2 from from chimpanzees to humans.”
“The methods of this paper have strikingly important implications for the study of pathogen transmission and the appearance of potential epidemics, as it is able to predict, given a specific set of environmental and demographic/population-based data, a jump from wildlife disease to human,” she wrote. “Better yet, it is able to predict potential intermediate hosts, within the context of the evolutionary history of both the hosts and the pathogens.”
Join the conversation
Corthals, who was not involved with the new research, said caution should be taken to ensure that the probability isn’t overcomplicated because of the huge data set and amount of possible variables.
“Should the methods stand further scrutiny and data-modeling, (this) will have serious implications in how we model and prevent pandemics,” Corthals said.
The research builds on a study from 2014 on the origin of human herpes viruses.
“We found that HSV-2 likely jumped from the ancestor of chimpanzees into a human ancestor, but we left it at that,” wrote Joel Wertheim, assistant professor at the University of California, San Diego Department of Medicine and lead author of the 2014 study, in an email. “This study continues our research through an unexpected avenue, looking at the prehistorical forest geography. This is not a line of inquiry I would have anticipated, but it provides a textured layer to the story of HSV origins.”
The researchers are now working with colleagues in South Africa and the United States to learn how other ancient conditions, like human pubic lice, transmitted to modern humans.
“The usual obstacles of not enough fossils and archaeological preservation are always present, but also contribute to making the subject such a compelling one,” Houldcroft said.